Remember those mind-boggling chemistry quizzes in high school? The ones that made you question everything you thought you knew about atoms and molecules? Well, today we’re tackling a particular type of chemical entity: polyatomic ions. These fascinating groups of atoms, bound together with a net electrical charge, can be tricky to wrap your head around. But fear not! This article is your guide to understanding these charged molecules and conquering any polyatomic ions quiz that comes your way.

Image: www.bestphysicianlife.com
Picture this: you’re in the lab, surrounded by flasks and beakers, immersed in the world of chemical reactions. You’re working diligently, carefully mixing solutions and observing the transformations taking place. Suddenly, you run into an unfamiliar chemical formula – one that involves a group of atoms acting as a single entity with a charge! This is the realm of polyatomic ions, and mastering their behavior is essential for mastering chemistry.
Delving into the World of Polyatomic Ions
Polyatomic ions, as the name suggests, are groups of two or more atoms bonded together and carrying a net electrical charge, either positive (cations) or negative (anions). Unlike monatomic ions formed from single atoms, these groups of atoms behave as a single unit, carrying their charge collectively.
Imagine a group of friends sharing a plate of cookies. Each friend represents an atom, and the plate represents the molecule. However, instead of sharing equally, one friend takes a slightly bigger portion, creating a charge imbalance. This imbalance creates the charge on the polyatomic ion, making it more likely to react in various chemical reactions.
Understanding the Fundamentals
To unravel the complexities of these charged entities, we need to establish a solid foundation. Let’s break down the key concepts:
- Ion: An atom or group of atoms with a net electrical charge. Ions are formed when atoms gain or lose electrons, leading to an imbalance between the number of protons and electrons.
- Polyatomic Ion: A group of atoms bonded together with a net electrical charge, acting as a single unit.
- Cation: A positively charged ion, formed when an atom or group of atoms loses electrons.
- Anion: A negatively charged ion, formed when an atom or group of atoms gains electrons.
Examples of Polyatomic Ions
Let’s dive into some commonly encountered examples of polyatomic ions:
- Hydroxide Ion (OH-): This negatively charged group of one oxygen and one hydrogen atom is commonly found in bases like sodium hydroxide (NaOH).
- Nitrate Ion (NO3-): Consisting of one nitrogen and three oxygen atoms, the nitrate ion carries a negative charge, often found in fertilizers and explosives.
- Phosphate Ion (PO43-): This ion is a crucial component of DNA and ATP (adenosine triphosphate), the energy currency of cells.
- Sulfate Ion (SO42-): A negatively charged group of one sulfur and four oxygen atoms, commonly used in detergents and as a source of sulfur in fertilizers.
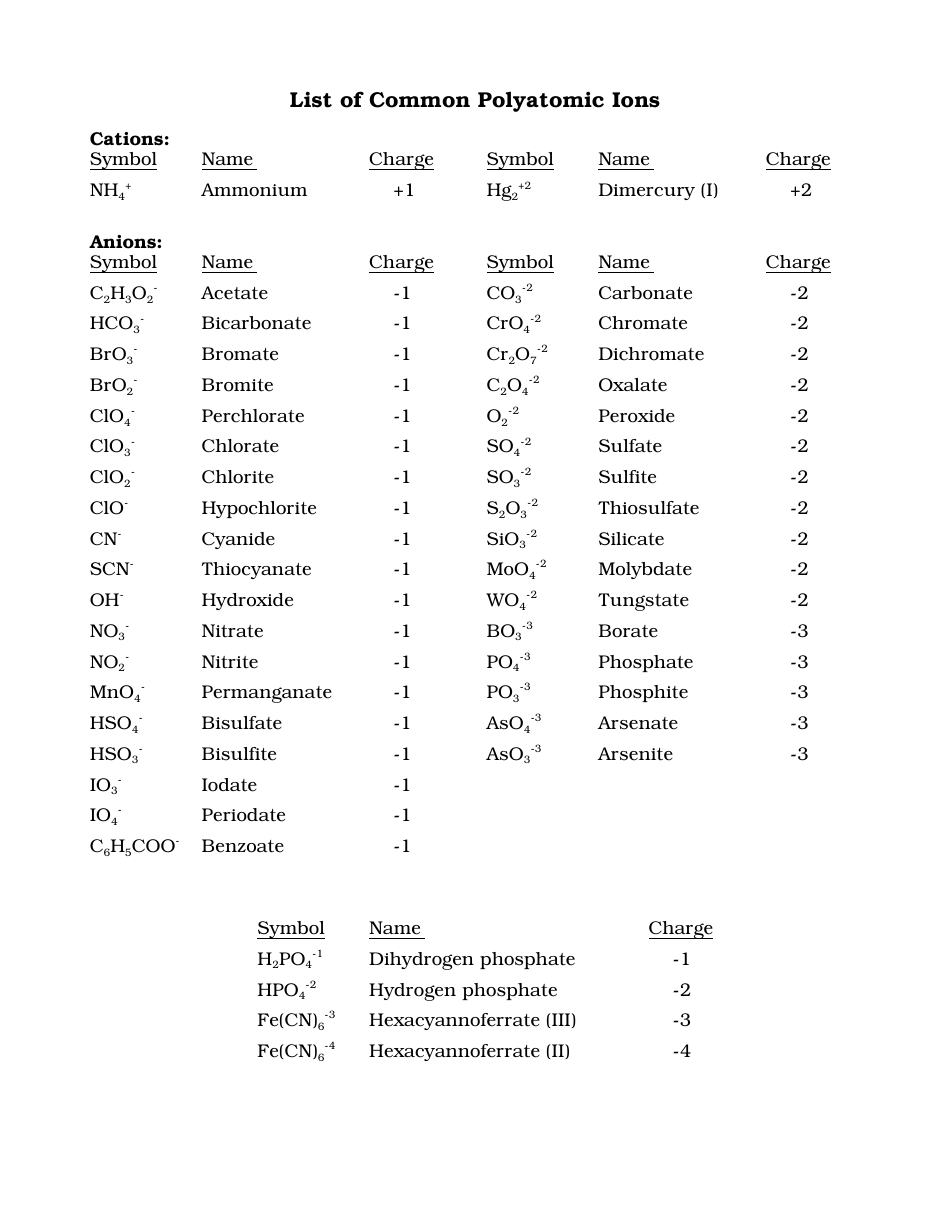
Image: www.templateroller.com
Polyatomic Ion Naming Conventions
The naming of polyatomic ions follows specific rules. Here’s a breakdown:
- Cations: Generally, the name of the metal cation is used, followed by the name of the polyatomic anion. For example, sodium hydroxide (NaOH) is named as sodium (Na+) + hydroxide (OH-).
- Anions: The names of polyatomic anions are often based on the nonmetal element they contain, with suffixes like “-ite” and “-ate” indicating the number of oxygen atoms and their oxidation state. For example, sulfite (SO32-) has less oxygen than sulfate (SO42-).
Testing Your Knowledge: The Polyatomic Ions Quiz
Now that you’ve gotten acquainted with the basics of polyatomic ions, it’s time to put your knowledge to the test! Here’s a quiz to solidify your understanding and help you prepare for any challenging chemistry exams.
Multiple Choice Questions
- Which of the following is NOT a polyatomic ion?
- Ammonium (NH4+)
- Chloride (Cl-)
- Carbonate (CO32-)
- Phosphate (PO43-)
- What is the name of the polyatomic ion with the formula SO42-?
- Sulfite
- Sulfate
- Sulphide
- Sulphur
- Which polyatomic ion is a component of DNA and ATP?
- Ammonium
- Phosphate
- Nitrate
- Sulfate
True or False
- Polyatomic ions are always negatively charged.
- The hydroxide ion (OH-) acts as a base.
- Polyatomic ions can be found in both ionic and covalent compounds.
- The name of a polyatomic ion always includes the prefix “poly-“.
Tips for Conquering the Polyatomic Ions Quiz
While polyatomic ions can be intimidating at first, with a few strategies, you can master them in no time. Here are some helpful tips:
- Create flashcards: Writing down the names and formulas of common polyatomic ions on flashcards can help you memorize them easily. Quiz yourself regularly to reinforce your learning.
- **Practice, practice, practice:** The more you encounter polyatomic ions in practice problems and quizzes, the more familiar you’ll become with their properties and how they behave in chemical reactions.
- **Visualize the structure:** Understanding the arrangement of atoms within a polyatomic ion can help you remember its name and formula. Draw diagrams or use online tools to visualize them.
- **Use mnemonic devices:** Create catchy phrases or rhymes to remember the names and formulas of polyatomic ions. This can be particularly helpful for challenging ions like sulfate and sulfite.
- **Understand the naming conventions: Mastering the naming rules for polyatomic ions is crucial for recognizing and identifying them correctly.
Expert Advice From a Chemistry Guru
As a seasoned chemistry teacher, I emphasize the importance of understanding the underlying principles behind polyatomic ions. Don’t solely rely on memorization; instead, try to grasp the reasoning behind their charge, structure, and behavior in chemical reactions. This will equip you with the skills to approach any polyatomic ions quiz with confidence.
Beyond memorization, understanding how polyatomic ions function in different chemical environments is key to truly comprehending their significance. Explore real-world applications, from fertilizers and batteries to biological processes, to appreciate the vital roles these charged groups play.
Frequently Asked Questions (FAQs)
Here are some common questions about polyatomic ions.
Q: Why do polyatomic ions have a charge?
A: Polyatomic ions have a charge due to an imbalance between the number of protons and electrons within their structure. This imbalance can arise from the sharing of electrons between atoms, with one atom gaining a slight negative charge and the other a slight positive charge.
Q: Are all polyatomic ions negatively charged?
A: No, not all polyatomic ions are negatively charged. Some, like ammonium (NH4+), carry a positive charge.
Q: How can I distinguish between sulfate and sulfite ions?
A: Sulfate (SO42-) has four oxygen atoms, while sulfite (SO32-) has three oxygen atoms. Remember that “-ate” indicates a higher oxidation state with more oxygen.
Q: Where can I find more information about polyatomic ions?
A: There are plenty of resources available online and in textbooks to learn more about polyatomic ions. Search for “polyatomic ions,” “chemistry textbook,” or “chemistry online resources” to find comprehensive information and practice problems.
Polyatomic Ions Quiz
Conclusion
Polyatomic ions, with their intricate structures and unique properties, play a crucial role in chemistry and our world. By mastering the concepts and applying the tips provided, you can overcome any polyatomic ions quiz and achieve mastery in chemistry. Remember, understanding the “why” behind the “what” will make your learning journey more engaging and effective.
Are you interested in learning more about polyatomic ions and their fascinating applications in different fields?






