The world of chemistry is an intricate dance of elements, each with its unique properties and quirks. Among these, sulfur stands out as a fascinating element. As a kid, I remember being captivated by the vibrant yellow color of sulfur crystals. What I didn’t know then was the depth of its chemical significance, particularly its molar mass. The molar mass of an element is a vital concept in chemistry, allowing us to calculate the weight of individual atoms and molecules. This article delves into the fascinating realm of sulfur’s molar mass, exploring its definition, significance, and relevance in various applications.
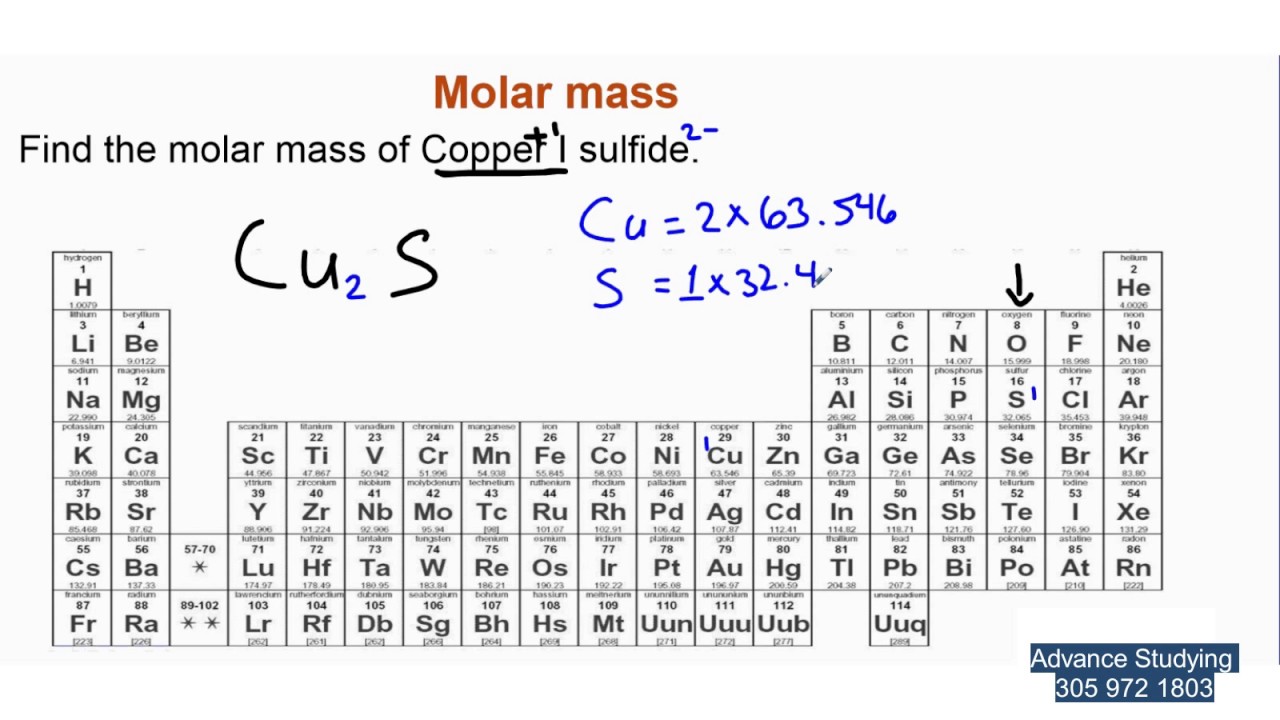
Image: 123bike.biz
From understanding chemical reactions to producing fertilizers and pharmaceuticals, knowledge of sulfur’s molar mass is paramount. This article will serve as your guide to unraveling the mysteries surrounding this essential aspect of sulfur.
Exploring the Molar Mass of Sulfur
The molar mass of an element represents the mass of one mole of its atoms. In simpler terms, it’s the weight of 6.022 x 10^23 atoms of that element. This number, known as Avogadro’s number, is a fundamental constant in chemistry. So, the molar mass of sulfur (S) is the mass of 6.022 x 10^23 sulfur atoms.
To determine the molar mass of sulfur, we look at its atomic weight on the periodic table. Sulfur’s atomic weight is approximately 32.065 atomic mass units (amu). The atomic mass unit is a standard unit of mass used for atoms and molecules. Since one amu is approximately equal to 1 gram per mole (g/mol), the molar mass of sulfur is approximately 32.065 g/mol.
Importance of Molar Mass in Chemistry
The molar mass of sulfur is a crucial factor in numerous chemical calculations, including:
1. Stoichiometry:
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. The molar mass of sulfur allows us to accurately calculate the amount of reactants needed or products produced in a specific reaction.
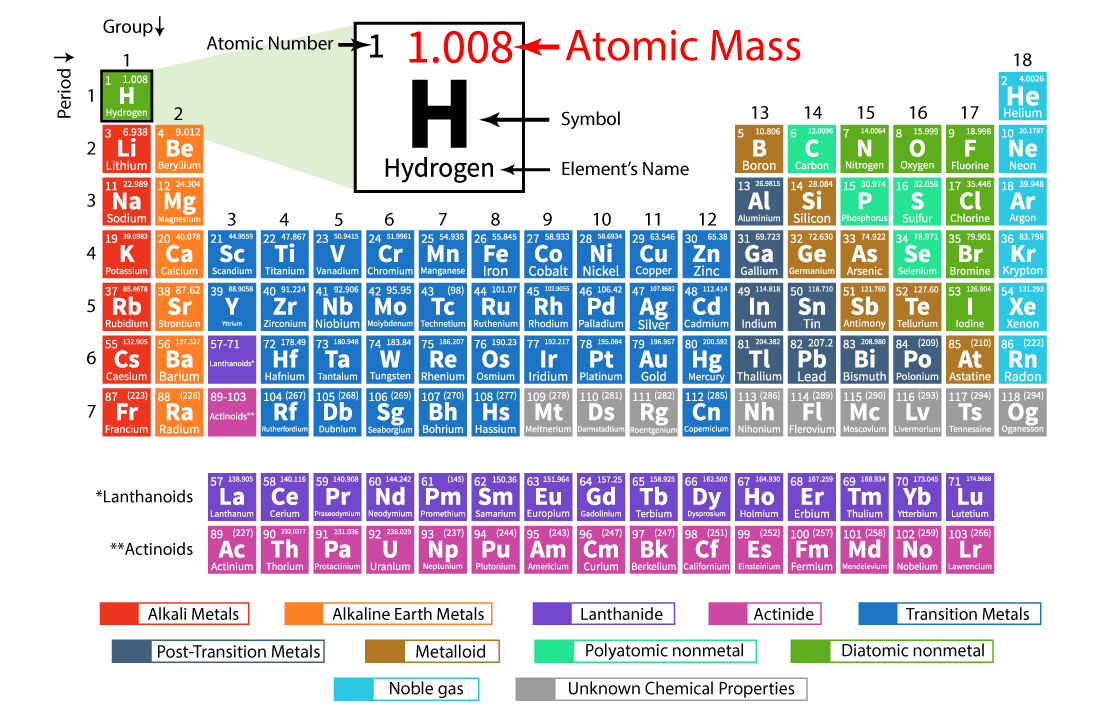
Image: tinnongtuyensinh.com
2. Concentration Calculations:
Molarity, a commonly used unit of concentration, represents the number of moles of a solute per liter of solution. To determine the molarity of a sulfur-containing solution, we need to know the molar mass of sulfur.
3. Mole Conversions:
Molar mass acts as a conversion factor between mass and moles. It allows us to convert a given mass of sulfur into the corresponding number of moles and vice versa. This is essential for various chemical calculations.
Trends and Developments in Sulfur Chemistry
Research and advancements in sulfur chemistry are ongoing, impacting various industries and fields.
1. Sulfur-based Batteries:
Sulfur is an essential component of lithium-sulfur batteries, a promising technology for electric vehicles and energy storage. These batteries offer higher energy densities and improved safety compared to conventional lithium-ion batteries. Advancements in sulfur chemistry are focusing on enhancing the performance and stability of these batteries.
2. Sulfur in Pharmaceuticals:
Sulfur plays a vital role in synthesizing various pharmaceuticals, including antibiotics, anti-inflammatory drugs, and antifungal agents. Research continues to explore novel applications of sulfur-containing compounds in the pharmaceutical industry, leading to the development of more effective and targeted medications.
3. Sulfur in Agriculture:
Sulfur is an essential nutrient for plant growth, supporting the synthesis of proteins and chlorophyll. Sulfur-based fertilizers are widely used to enhance crop yield and quality. Research is exploring sustainable ways to utilize sulfur in agriculture, promoting environmentally friendly practices.
Tips for Working with Sulfur
Working with sulfur requires safety precautions due to its potential for irritation and toxicity. Here are some tips:
1. Proper Handling:
Always wear appropriate personal protective equipment (PPE), including gloves, goggles, and a respirator when handling sulfur or sulfur-containing compounds. Avoid direct contact with skin, eyes, and respiratory system.
2. Adequate Ventilation:
Work in a well-ventilated area to minimize exposure to sulfur fumes. Ensure proper ventilation systems are in place and avoid working in confined spaces.
3. Storage Guidelines:
Store sulfur in tightly sealed containers in a cool, dry place away from direct sunlight and heat sources. Follow proper storage guidelines to prevent contamination or safety hazards.
FAQs about Molar Mass of Sulfur
Q: What is the difference between atomic mass and molar mass?
Atomic mass refers to the mass of a single atom of an element, while molar mass refers to the mass of one mole of atoms, which is 6.022 x 10^23 atoms. Atomic mass units (amu) are used for individual atoms, while g/mol is used for molar masses.
Q: How is the molar mass of sulfur used in calculations?
The molar mass of sulfur acts as a conversion factor between mass (in grams) and moles. It allows us to calculate the number of moles of sulfur in a given mass or the mass of a given number of moles. For example, if we have 16 grams of sulfur, we can divide it by the molar mass (32.065 g/mol) to get the number of moles (0.5 moles).
Q: What are some real-world applications of sulfur and its molar mass?
Sulfur is used in a wide range of applications, including the production of sulfuric acid, fertilizers, pesticides, and pharmaceuticals. The molar mass of sulfur is essential for calculating the amount of sulfur needed in various industrial processes, ensuring accurate measurements and optimal results.
Molar Mass Of S
Conclusion
Understanding the molar mass of sulfur is crucial for various chemical calculations, from stoichiometry to concentration determination. Its importance transcends laboratory settings, impacting various industrial applications, from energy storage to agriculture and pharmaceuticals. As research continues to unravel the complexities of sulfur chemistry, the knowledge of its molar mass will play a pivotal role in driving advancements in these fields.
Are you interested in learning more about sulfur’s role in different industries? Share your thoughts and questions in the comments section below!






