Have you ever wondered how seemingly opposite forces attract each other? In the world of chemistry, this fascinating phenomenon plays out on an atomic level, where a mesmerizing dance of electrons forms the foundation of countless compounds. This is the world of ionic bonds, a type of chemical bond where one atom completely donates an electron to another, leading to the formation of stable ions. But even within this realm of ionic interactions, a special kind of bond emerges, one characterized by a unique electron sharing mechanism – the coordinate ionic bond.
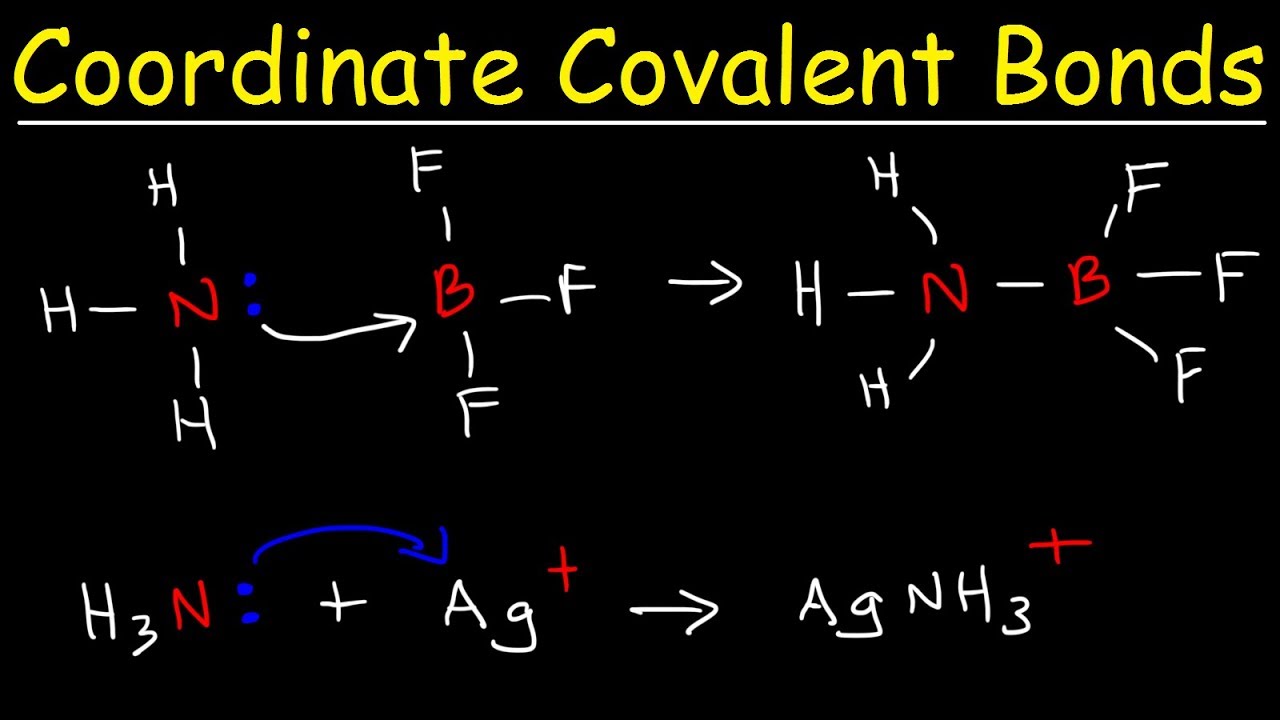
Image: mungfali.com
Today, we embark on a journey to unravel the mystery of this special type of bond, exploring its origins, characteristics, and significance in the vast tapestry of chemical reactions. Understanding the nature of coordinate ionic bonds unlocks the secrets behind the formation of complex molecules and expands our comprehension of the intricate interplay between atoms. So, buckle up and get ready to delve into the fascinating world of coordinate ionic bonds!
The Building Blocks of Bonding: A Primer on Ionic Bonds
To truly appreciate the uniqueness of coordinate ionic bonds, we need to first understand the fundamentals of ionic bonding. In essence, ionic bonds arise from the electrostatic attraction between oppositely charged ions. These ions are formed when an atom loses or gains electrons, resulting in a net positive or negative charge, respectively.
Consider the classic example of sodium chloride (NaCl), common table salt. Sodium (Na) has one valence electron, meaning it occupies the outermost energy level. Chlorine (Cl), on the other hand, has seven valence electrons. In order to achieve a stable electron configuration, sodium readily donates its single valence electron to chlorine. This process transforms sodium into a positively charged ion (Na+) and chlorine into a negatively charged ion (Cl-). The electrostatic attraction between these oppositely charged ions forms the ionic bond that holds sodium chloride together.
Coordinate Ionic Bonds: A Shared Dance of Electrons
Now, let’s introduce the captivating world of coordinate ionic bonds. Unlike regular ionic bonds where one atom completely transfers an electron to another, coordinate ionic bonds are characterized by the donation of a lone pair of electrons from one atom to the empty orbital of another atom. It’s like a shared dance where one partner provides the music and the other partner does the dance!
To visualize this better, imagine a molecule of ammonia (NH3). The nitrogen atom in ammonia has a lone pair of electrons in its outermost shell. When ammonia interacts with a proton (H+), the lone pair of electrons on nitrogen is donated to the empty orbital of the proton. This donation creates a new bond, a coordinate covalent bond, between the nitrogen atom and the proton, resulting in the formation of an ammonium ion (NH4+).
Key Characteristics of Coordinate Ionic Bonds:
- Electron Donation: The defining characteristic of a coordinate ionic bond is the donation of a lone pair of electrons from one atom (donor atom) to another atom (acceptor atom) with an empty orbital.
- Formation of a New Bond: The donation of electrons creates a new chemical bond, resulting in a complex ion with a coordinate ionic bond.
- Stability: Coordinate ionic bonds, like other ionic bonds, contribute to the overall stability of the resulting complex by satisfying the octet rule, where atoms strive to have eight electrons in their outermost shell.
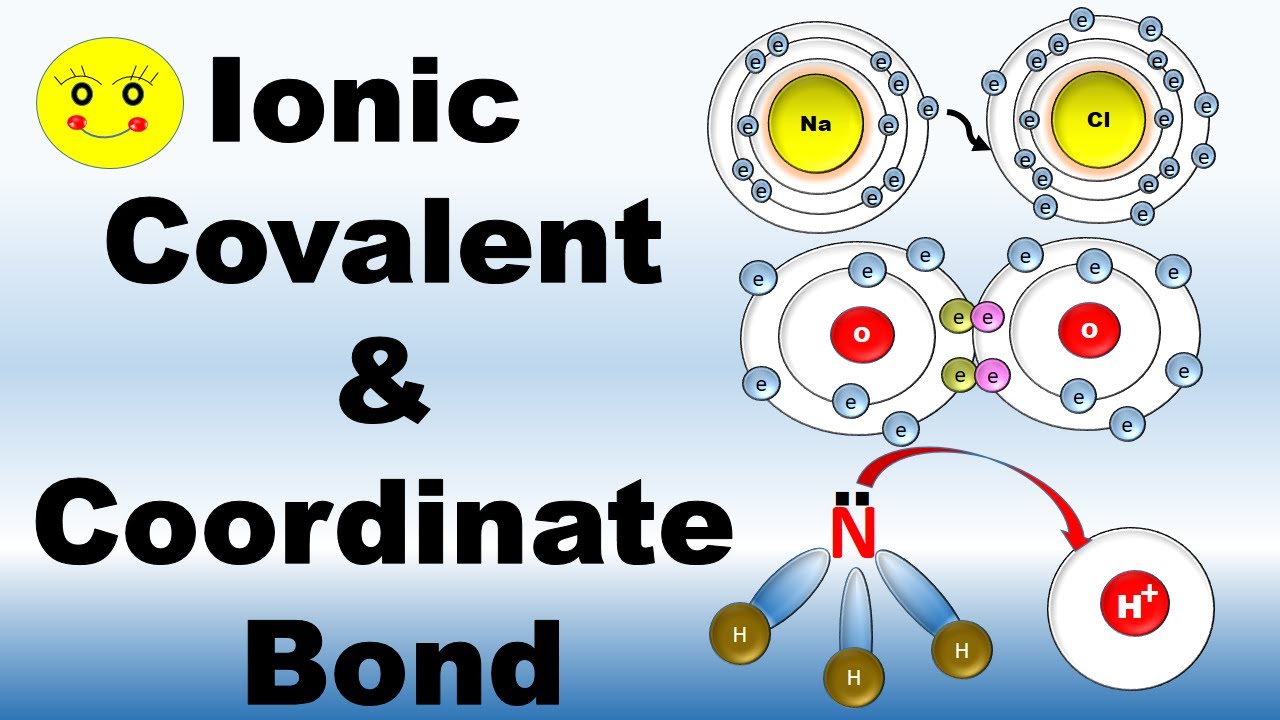
Image: www.myxxgirl.com
The Power of Coordination: Applications in Chemistry and Beyond
Coordinate ionic bonds play a vital role in numerous areas of chemistry, impacting diverse fields ranging from biochemistry to material science. Let’s explore some notable applications:
1. Complex Formation:
Coordinate ionic bonds are instrumental in the formation of coordination complexes. These complexes consist of a central metal ion surrounded by ligands, which are molecules or ions that donate electrons to the central metal ion through coordinate ionic bonds. Examples of coordination complexes include hemoglobin, responsible for transporting oxygen in our bloodstream, and chlorophyll, which facilitates photosynthesis in plants.
2. Acid-Base Chemistry:
Coordinate ionic bonds are central to the concept of Lewis acids and bases. Lewis acids are electron acceptors, while Lewis bases are electron donors. The formation of a coordinate ionic bond between a Lewis acid and a Lewis base is a classic example of a Lewis acid-base reaction. A common example is the reaction between ammonia, a Lewis base, and boron trifluoride, a Lewis acid, resulting in the formation of a complex with a coordinate ionic bond.
3. Catalysis:
Many catalytic reactions rely on the formation of coordinate ionic bonds. These bonds allow catalysts to bind to reactants, facilitating chemical reactions by lowering the activation energy. Transition metal complexes with coordinate ionic bonds are particularly important in catalysis, such as the use of platinum catalysts in the hydrogenation of alkenes.
4. Material Science:
Coordinate ionic bonds play a crucial role in developing advanced materials with unique properties. For instance, coordination polymers are a class of materials built from metal ions and organic ligands connected by coordinate ionic bonds. These polymers possess remarkable properties like high surface area, porosity, and electrical conductivity, making them ideal for applications in gas storage, catalysis, and electronics.
The Future of Coordinate Ionic Bonding: Unlocking New Frontiers
Research into coordinate ionic bonds continues to thrive, driven by the potential to synthesize novel materials with tailored properties. Scientists are exploring the use of coordinate ionic bonds to develop next-generation batteries with higher energy density and improved charge-discharge rates. This advancement is crucial for meeting the growing demand for energy storage in electric vehicles and portable electronics.
Another exciting area of research involves utilizing coordinate ionic bonds to create biocompatible materials for drug delivery and tissue engineering. The ability to control the formation and properties of coordinate ionic bonds allows researchers to design materials that can mimic the intricate structure and function of biological systems.
Coordinate Ionic Bond
Bridging the Gap: A Summary and Call to Action
The coordinate ionic bond, a unique type of bond characterized by the donation of a lone pair of electrons, is a fundamental concept in chemistry with profound implications for various fields. We’ve explored its definition, key characteristics, and its key role in complex formation, acid-base chemistry, catalysis, and material science. Today, research on coordinate ionic bonds is being fueled by the quest for novel materials and technologies, unlocking doors to new frontiers in energy storage, drug delivery, and biocompatible materials.
So, continue to explore the fascinating world of coordinate ionic bonds. Read further about their diverse applications and delve into the intricacies of the electron dance that forms their foundation. Remember, understanding these bonds unlocks a deeper appreciation for the intricate world of chemistry, where shared love between atoms ultimately builds the world around us.






